Outline ·
[ Standard ] ·
Linear+
I dont understand the 2nd law of thermodynamics
|
TSGrammar Police
|
 Jul 28 2021, 12:42 PM, updated 5y ago Jul 28 2021, 12:42 PM, updated 5y ago
|

|
i read many diff explanations but still I dont understand the 2nd law of thermodynamics.  Wiki : The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. Entropy predicts the direction of spontaneous processes, and determines whether they are irreversible or impossible, despite obeying the requirement of conservation of energy, which is established in the first law of thermodynamics. Nasa : 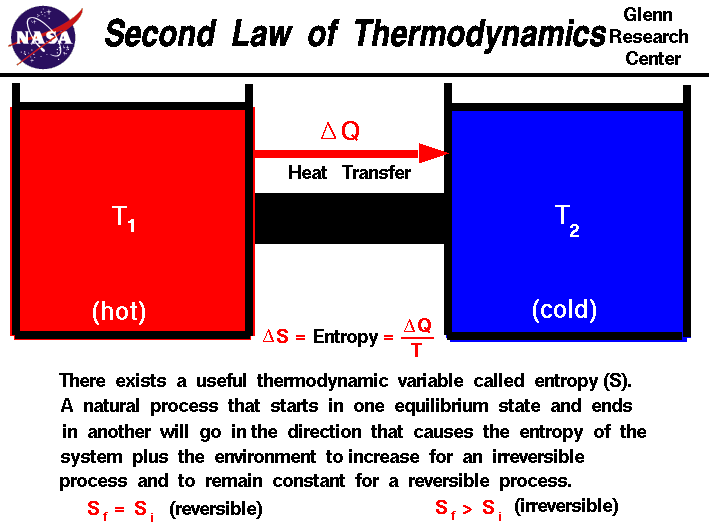 livescience : The Second Law of Thermodynamics is about the quality of energy. It states that as energy is transferred or transformed, more and more of it is wasted. The Second Law also states that there is a natural tendency of any isolated system to degenerate into a more disordered state. I knw /k many pandai, can care to explain to me? |
|
|
|
|
|
alexander3133
|
 Jul 28 2021, 12:47 PM Jul 28 2021, 12:47 PM
|

|
|
|
|
|
|
|
zack85
|
 Jul 28 2021, 12:50 PM Jul 28 2021, 12:50 PM
|
New Member


|
QUOTE(Grammar Police @ Jul 28 2021, 12:42 PM) i read many diff explanations but still I dont understand the 2nd law of thermodynamics.  Wiki : The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. Entropy predicts the direction of spontaneous processes, and determines whether they are irreversible or impossible, despite obeying the requirement of conservation of energy, which is established in the first law of thermodynamics. Nasa :  livescience : The Second Law of Thermodynamics is about the quality of energy. It states that as energy is transferred or transformed, more and more of it is wasted. The Second Law also states that there is a natural tendency of any isolated system to degenerate into a more disordered state. I knw /k many pandai, can care to explain to me? i got D for thermodynamics and still become an engineer...so wat tak pedulik je...  |
|
|
|
|
|
feynman
|
 Jul 28 2021, 12:50 PM Jul 28 2021, 12:50 PM
|
Look at all my stars!!


|
pasar explanation....
heat is low "quality energy", once energy is in this form, you can't convert it back to say nuclear energy.........
which also means, the universe will eventually be a very cold place................the sun and all the stars will expend all its energy through fusion, giving out heat, light, magnetic energies.....once gone, then that's it. No more work can be done.....
|
|
|
|
|
|
feynman
|
 Jul 28 2021, 12:50 PM Jul 28 2021, 12:50 PM
|
Look at all my stars!!


|
pasar explanation....
heat is low "quality energy", once energy is in this form, you can't convert it back to say nuclear energy.........
which also means, the universe will eventually be a very cold place................the sun and all the stars will expend all its energy through fusion, giving out heat, light, magnetic energies.....once gone, then that's it. No more work can be done.....
|
|
|
|
|
|
Kylow
|
 Jul 28 2021, 12:51 PM Jul 28 2021, 12:51 PM
|
Getting Started
 

|
You aerospace?
If no just pass enough
All da best
|
|
|
|
|
|
SUSgsem984
|
 Jul 28 2021, 12:52 PM Jul 28 2021, 12:52 PM
|
Getting Started
 

|
it is self explanatory like simple english only. which part you don't understand?
|
|
|
|
|
|
TSGrammar Police
|
 Jul 28 2021, 12:52 PM Jul 28 2021, 12:52 PM
|

|
QUOTE(feynman @ Jul 28 2021, 12:50 PM) pasar explanation.... heat is low "quality energy", once energy is in this form, you can't convert it back to say nuclear energy......... which also means, the universe will eventually be a very cold place................the sun and all the stars will expend all its energy through fusion, giving out heat, light, magnetic energies.....once gone, then that's it. No more work can be done..... but the first law of thermodynamics say energy cannot be destroyed so the energy star gives out wont be destroyed or disappeared, it will still be there in one form or another, lingering around This post has been edited by Grammar Police: Jul 28 2021, 12:59 PM |
|
|
|
|
|
billylks
|
 Jul 28 2021, 12:52 PM Jul 28 2021, 12:52 PM
|
Getting Started
 

|
Last time I studied Physic in Malay. When I read TS's paragraphs I don't understand a thing, lol.
|
|
|
|
|
|
tomatotomatomy
|
 Jul 28 2021, 12:53 PM Jul 28 2021, 12:53 PM
|
Getting Started
 

|
It means that energy without any intervention, will always degrade/decay rom higher order to lower order.
Think of the sun, one day after millions of years, all it’s energy will be lost as heat and light to the universe.
|
|
|
|
|
|
Zyrise5465
|
 Jul 28 2021, 12:54 PM Jul 28 2021, 12:54 PM
|
Getting Started
 

|
Entropy, enthalpy.. heat sink that sink. All return bak to lecturar..
|
|
|
|
|
|
demongoh
|
 Jul 28 2021, 12:55 PM Jul 28 2021, 12:55 PM
|
Getting Started
 

|
try this youtube link to get an understanding of the 2nd law https://www.youtube.com/watch?v=iWgKxZIA2uA |
|
|
|
|
|
J1g54w
|
 Jul 28 2021, 12:55 PM Jul 28 2021, 12:55 PM
|

|
QUOTE(Grammar Police @ Jul 28 2021, 12:52 PM) but the first law of thermodynamics say energy cannot be destroyed so the energy star gives out wont be destroyed or disappeared, it will still be there in one form or another 2nd law only applies in a closed system |
|
|
|
|
|
Clea
|
 Jul 28 2021, 01:00 PM Jul 28 2021, 01:00 PM
|
New Member


|
you have a deck of cards arranged from 1 to 1000 and you shuffle the deck. Then you check the cards one by one to see how they are numbered. The probability of it again being numbered 1 to 1000 (ordered) nicely is far lower than the probability of it being a jumbled mess (disorder). All systems tend to disorder ( and this is interestingly kind of an indication of the direction of the arrow of time)
About energy, consider this: energy cannot be created or destroyed. But then why dont we have unlimited energy because energy cannot be destroyed? The thing is each form of energy has a different 'quality' or 'order' to it. The lowest quality energy you can get (I think) is ambient heat. You cannot, even in theory, create a machine that can extract ambient heat from the environment to power your whatever devices and cool down the environment at the same time. There must be a difference in heat for energy extraction to be possible. So lets say you have a light bulb. You convert electricity (high order) to light (lower order). The light then becomes heat in the end (lowest order) and equilibrize with the environment heat. Now you cannot extract the same heat from the environment to convert into electricity to power your light bulb as before.
edit
This post has been edited by Clea: Jul 28 2021, 01:01 PM
|
|
|
|
|
|
andyng38
|
 Jul 28 2021, 01:00 PM Jul 28 2021, 01:00 PM
|

|
U can write to Tun M for an explanation. Err nevermind... he only knows Thanosdynamics.
|
|
|
|
|
|
jibpek
|
 Jul 28 2021, 01:00 PM Jul 28 2021, 01:00 PM
|

|
Conservation of energy says that the total energy within a closed system remains the same.
Entropy says that the quality of energy (whether it can be utilized).
Entropy always increases
|
|
|
|
|
|
accitzone
|
 Jul 28 2021, 01:02 PM Jul 28 2021, 01:02 PM
|

|
QUOTE(zack85 @ Jul 28 2021, 12:50 PM) i got D for thermodynamics and still become an engineer...so wat tak pedulik je...  where do you work? gonna mark your company out of any potential list |
|
|
|
|
|
TSGrammar Police
|
 Jul 28 2021, 01:06 PM Jul 28 2021, 01:06 PM
|

|
QUOTE(demongoh @ Jul 28 2021, 12:55 PM) try this youtube link to get an understanding of the 2nd law https://www.youtube.com/watch?v=iWgKxZIA2uAi understand nao. thank u and feynmanThis post has been edited by Grammar Police: Jul 28 2021, 01:06 PM |
|
|
|
|
|
Nama saya Amad
|
 Jul 28 2021, 01:06 PM Jul 28 2021, 01:06 PM
|

|
QUOTE(zack85 @ Jul 28 2021, 12:50 PM) i got D for thermodynamics and still become an engineer...so wat tak pedulik je...  No wonder kilang terbakar, jambatan runtuh trolololik |
|
|
|
|
|
mushigen
|
 Jul 28 2021, 01:06 PM Jul 28 2021, 01:06 PM
|

|
QUOTE(billylks @ Jul 28 2021, 12:52 PM) Last time I studied Physic in Malay. When I read TS's paragraphs I don't understand a thing, lol. Ha, I too had a tough time with the terms in English (force, acceleration, etc). I had this issue too, in Chemistry. Took me a while to get used to calling "mangkin" catalyst. And always had a hard time distinguishing sodium and potassium (called natrium and kalium). |
|
|
|
|
|
hotdayum
|
 Jul 28 2021, 01:08 PM Jul 28 2021, 01:08 PM
|
Getting Started
 

|
QUOTE(Grammar Police @ Jul 28 2021, 12:52 PM) but the first law of thermodynamics say energy cannot be destroyed so the energy star gives out wont be destroyed or disappeared, it will still be there in one form or another, lingering around Entropy is energy also. Just energy you kenot use. |
|
|
|
|
|
TSGrammar Police
|
 Jul 28 2021, 01:11 PM Jul 28 2021, 01:11 PM
|

|
QUOTE(hotdayum @ Jul 28 2021, 01:08 PM) Entropy is energy also. Just energy you kenot use. sad! need a new big bang to reset the entropy |
|
|
|
|
|
feynman
|
 Jul 28 2021, 01:12 PM Jul 28 2021, 01:12 PM
|
Look at all my stars!!


|
QUOTE(Grammar Police @ Jul 28 2021, 12:52 PM) but the first law of thermodynamics say energy cannot be destroyed so the energy star gives out wont be destroyed or disappeared, it will still be there in one form or another a photon will still be a photon in space until it interacts with matter What potential energy the star has would have been converted to all other forms of energy, making the star devoid of anything.....it would just be a hunk of matter approaching zero kelvins it will just be a boring place. If you look at stars that are towards the end of their lives...the fact that radio telescopes can still see them tells you that they are still dimly radiating EM waves, eventually that will also stop and it won't be detectable. |
|
|
|
|
|
hey_nello
|
 Jul 28 2021, 01:12 PM Jul 28 2021, 01:12 PM
|
Getting Started
 

|
Heat disperses, Energy disperses - example hot cocoa becomes cooler
To collect back the energy also must use energy(disperse more energy) - example to heat back the cocoa u must use electricity(electricity is produced by dispersing energy - burning fossil fuel or the sun)
So in total the universe is dispersing energy until it become meaningless dispersed energy in empty space. (Cold death)
If u reverse this timeline u get will get the big bang - the universe starts out with the lowest entropy (highly arranged and efficient form, NOT random and messy) thus proving the existence of GOD..
|
|
|
|
|
|
hotdayum
|
 Jul 28 2021, 01:14 PM Jul 28 2021, 01:14 PM
|
Getting Started
 

|
QUOTE(Grammar Police @ Jul 28 2021, 01:11 PM) sad! need a new big bang to reset the entropy Theoretically that requires everything repeat everything to go back into a single point. |
|
|
|
|
|
feynman
|
 Jul 28 2021, 01:21 PM Jul 28 2021, 01:21 PM
|
Look at all my stars!!


|
QUOTE(Grammar Police @ Jul 28 2021, 01:06 PM) i understand nao. thank u and feynmankey word is work........energy and work have the same physical units of course...that's your law. Thermal physics became a discipline in the 1800s because of the industrial revolution. Engineers and physicists formulated ways to exploit energy through machines to do work and very rapidly, they understood that the "process" only/mostly moves one way in order for work to be done. of course, you can still reverse the process but that would entail doing useful work on the system to restore it in its original state......so really, doesn't make economic or physical sense to do so. |
|
|
|
|
|
zack85
|
 Jul 28 2021, 01:24 PM Jul 28 2021, 01:24 PM
|
New Member


|
QUOTE(accitzone @ Jul 28 2021, 01:02 PM) where do you work? gonna mark your company out of any potential list u dont cb..    |
|
|
|
|
|
zack85
|
 Jul 28 2021, 01:25 PM Jul 28 2021, 01:25 PM
|
New Member


|
QUOTE(Nama saya Amad @ Jul 28 2021, 01:06 PM) No wonder kilang terbakar, jambatan runtuh trolololik my company non-related to kilang terbakar and jambatan runtuh   |
|
|
|
|
|
tomatotomatomy
|
 Jul 28 2021, 01:26 PM Jul 28 2021, 01:26 PM
|
Getting Started
 

|
Wow kopitiam so many physicists
We have so many talents in Malaysia
When talk about MH170, all the aeronautics engineers come out
When talk about COVID, all the epidemiologists and virologists come out
When talk about thermodynamics, all the nasa engineers come out
|
|
|
|
|
|
zack85
|
 Jul 28 2021, 01:28 PM Jul 28 2021, 01:28 PM
|
New Member


|
QUOTE(tomatotomatomy @ Jul 28 2021, 01:26 PM) Wow kopitiam so many physicists We have so many talents in Malaysia When talk about MH170, all the aeronautics engineers come out When talk about COVID, all the epidemiologists and virologists come out When talk about thermodynamics, all the nasa engineers come out when talk about work implementation...all cabut lari  |
|
|
|
|
|
feekle
|
 Jul 28 2021, 01:30 PM Jul 28 2021, 01:30 PM
|

|
Dont go too deep..religion doesnt like it
|
|
|
|
|
|
zeese
|
 Jul 28 2021, 01:31 PM Jul 28 2021, 01:31 PM
|

|
F that S. You'll never use it in your work anyway..
|
|
|
|
|
|
SUSCmyong88
|
 Jul 28 2021, 01:34 PM Jul 28 2021, 01:34 PM
|

|
You cannot eat your poop to self sustain.
|
|
|
|
|
|
TSGrammar Police
|
 Jul 28 2021, 01:36 PM Jul 28 2021, 01:36 PM
|

|
QUOTE(hotdayum @ Jul 28 2021, 01:14 PM) Theoretically that requires everything repeat everything to go back into a single point. wait dont new stars get born every now and then? gotta google that up |
|
|
|
|
|
eidrag
|
 Jul 28 2021, 01:37 PM Jul 28 2021, 01:37 PM
|
Getting Started
 

|
it's like universe getting colder every time /k members open story
|
|
|
|
|
|
hotdayum
|
 Jul 28 2021, 01:38 PM Jul 28 2021, 01:38 PM
|
Getting Started
 

|
QUOTE(Grammar Police @ Jul 28 2021, 01:36 PM) wait dont new stars get born every now and then? gotta google that up That's not single point leh. Stars are born when enough mass come together that fusion begins. |
|
|
|
|
|
thickface
|
 Jul 28 2021, 01:38 PM Jul 28 2021, 01:38 PM
|
Getting Started
 

|
Best example is the aircond.
Think of it this way, if real world scenario, you would use aircond to cool down a room and reduce the entropy in the room. However, the energy to power up the aircond will generate more entropy outside the room compared to reduction of entropy in room. Sum up, you still create more entropy. Thus, final is higher than initial (which is bad).
|
|
|
|
|
|
TSGrammar Police
|
 Jul 28 2021, 01:39 PM Jul 28 2021, 01:39 PM
|

|
QUOTE(hotdayum @ Jul 28 2021, 01:38 PM) That's not single point leh. Stars are born when enough mass come together that fusion begins. Star Formation Stars are born within the clouds of dust and scattered throughout most galaxies. A familiar example of such as a dust cloud is the Orion Nebula. Turbulence deep within these clouds gives rise to knots with sufficient mass that the gas and dust can begin to collapse under its own gravitational attraction. As the cloud collapses, the material at the center begins to heat up. Known as a protostar, it is this hot core at the heart of the collapsing cloud that will one day become a star. Three-dimensional computer models of star formation predict that the spinning clouds of collapsing gas and dust may break up into two or three blobs; this would explain why the majority the stars in the Milky Way are paired or in groups of multiple stars. NASA https://science.nasa.gov/astrophysics/focus...form-and-evolveso universe is not going to die a cold death because new stars get borned... |
|
|
|
|
|
Gon Freaks
|
 Jul 28 2021, 01:40 PM Jul 28 2021, 01:40 PM
|

|
QUOTE(Grammar Police @ Jul 28 2021, 12:52 PM) but the first law of thermodynamics say energy cannot be destroyed so the energy star gives out wont be destroyed or disappeared, it will still be there in one form or another, lingering around the second law never said that energy is destroyed, but rather transferred and change it form to a more passive energy. |
|
|
|
|
|
hotdayum
|
 Jul 28 2021, 01:44 PM Jul 28 2021, 01:44 PM
|
Getting Started
 

|
QUOTE(Grammar Police @ Jul 28 2021, 01:39 PM) Star Formation Stars are born within the clouds of dust and scattered throughout most galaxies. A familiar example of such as a dust cloud is the Orion Nebula. Turbulence deep within these clouds gives rise to knots with sufficient mass that the gas and dust can begin to collapse under its own gravitational attraction. As the cloud collapses, the material at the center begins to heat up. Known as a protostar, it is this hot core at the heart of the collapsing cloud that will one day become a star. Three-dimensional computer models of star formation predict that the spinning clouds of collapsing gas and dust may break up into two or three blobs; this would explain why the majority the stars in the Milky Way are paired or in groups of multiple stars. NASA https://science.nasa.gov/astrophysics/focus...form-and-evolveso universe is not going to die a cold death because new stars get borned... This is where the 2nd Law comes in. The fusion in stars partially converts to entropy, which just stays in the background. Other things absorb the resulting heat and radiation, their atoms vibrate, further converting some of that energy to entropy. |
|
|
|
|



 Jul 28 2021, 12:42 PM, updated 5y ago
Jul 28 2021, 12:42 PM, updated 5y ago
 Quote
Quote
 0.0232sec
0.0232sec
 0.34
0.34
 5 queries
5 queries
 GZIP Disabled
GZIP Disabled