Outline ·
[ Standard ] ·
Linear+
Chemistry A few questions, need help please
|
TSCKC_1
|
 Jul 19 2009, 12:49 AM, updated 17y ago Jul 19 2009, 12:49 AM, updated 17y ago
|

|
I have a few questions in my chemistry book that I do not understand enough to move on to the next section . Can someone help me?
Reactions rates are affected by concentration ,collision geometry , and the presence of a catalyst . Which of the following statement is false concerning these factors.
a Increasing the concentration of reacting particles increases the chance for collision
b Optimum collision geometry lowers the activation energy barrier
c A reaction occurs each time particles of the reactants collide.
d The slowest reaction incolved in a reaction mechanism determines the rate of overall reaction
the correct answer is c ,but why? I did alittle research online and found this statement "A chemical reaction occurs when substances (the reactants) collide with enough energy to rearrange to form different compounds (the products)."
isnt d correct" why then is c false?
Can someone also explain B and D?
Another question :
I needed to do this calculation :
I needed to balance the equation first (which i did already) and calculate ΔH
2(C2H6) + 7(O2) ---> 4(CO2) + 6(H2O) <<already balanced
C-H = 99kcal
O-O = 119kcal
C-C = 83.1kcal
C=O = 192kcal
O-H = 111kcal
ΔH = ??
I did the calculation this way
Products = C-H 2( 99*6) + C-C 2(83.1) + O-O 7(119) = 2187.2 kcal
Reactants = O=C 4(192*2) + H-O 6(111*2) = -2868
2187.2 + (-2868) = -680.8 kcal
The correct answer was -340.4 kcal
Why do i have to divide by 2?
|
|
|
|
|
|
0606088
|
 Jul 19 2009, 01:23 AM Jul 19 2009, 01:23 AM
|

|
A reaction occurs each time particles of the reactants collide.
- each time particles of the reactants collide couldn't be everytime also a sucess reaction (form product). another factor to be consider is collision geometry, enough energy and etc.
Optimum collision geometry lowers the activation energy barrier.
- when there is the right geometry of collision, the reaction will be happened immediately without need of energy (higher temperature). this will lowers the activation energy.
The slowest reaction incolved in a reaction mechanism determines the rate of overall reaction.
- the slowest is the determines the rate because we only could calculate the rate of reaction when all the reaction is finish. meaning that there is no reactant remaining in the reaction.
|
|
|
|
|
|
TSCKC_1
|
 Jul 19 2009, 02:20 PM Jul 19 2009, 02:20 PM
|

|
thank you so much , do you know how to do the other one?
|
|
|
|
|
|
befitozi
|
 Jul 19 2009, 04:30 PM Jul 19 2009, 04:30 PM
|

|
QUOTE(CKC_1 @ Jul 19 2009, 12:49 AM) Another question : I needed to do this calculation : I needed to balance the equation first (which i did already) and calculate ΔH 2(C2H6) + 7(O2) ---> 4(CO2) + 6(H2O) <<already balanced C-H = 99kcal
O-O = 119kcal
C-C = 83.1kcal
C=O = 192kcal
O-H = 111kcal
ΔH = ??
I did the calculation this way
Products = C-H 2( 99*6) + C-C 2(83.1) + O-O 7(119) = 2187.2 kcal
Reactants = O=C 4(192*2) + H-O 6(111*2) = -2868
2187.2 + (-2868) = -680.8 kcal
The correct answer was -340.4 kcal
Why do i have to divide by 2?Shouldn't you be looking for energy of O=O bond instead of O-O? |
|
|
|
|
|
TSCKC_1
|
 Jul 19 2009, 05:28 PM Jul 19 2009, 05:28 PM
|

|
uhh , no , theres a graph in my book but i dont know how to draw the whole thing out..theres no O=O
|
|
|
|
|
|
vivienne85
|
 Jul 19 2009, 07:07 PM Jul 19 2009, 07:07 PM
|

|
QUOTE(CKC_1 @ Jul 19 2009, 12:49 AM) I have a few questions in my chemistry book that I do not understand enough to move on to the next section . Can someone help me? Reactions rates are affected by concentration ,collision geometry , and the presence of a catalyst . Which of the following statement is false concerning these factors.
a Increasing the concentration of reacting particles increases the chance for collision
b Optimum collision geometry lowers the activation energy barrier
c A reaction occurs each time particles of the reactants collide.
d The slowest reaction incolved in a reaction mechanism determines the rate of overall reactionthe correct answer is c ,but why? I did alittle research online and found this statement "A chemical reaction occurs when substances (the reactants) collide with enough energy to rearrange to form different compounds (the products)."
isnt d correct" why then is c false?
Can someone also explain B and D?Another question : I needed to do this calculation : I needed to balance the equation first (which i did already) and calculate ΔH 2(C2H6) + 7(O2) ---> 4(CO2) + 6(H2O) <<already balanced C-H = 99kcal
O-O = 119kcal
C-C = 83.1kcal
C=O = 192kcal
O-H = 111kcal
ΔH = ??
I did the calculation this way
Products = C-H 2( 99*6) + C-C 2(83.1) + O-O 7(119) = 2187.2 kcal
Reactants = O=C 4(192*2) + H-O 6(111*2) = -2868
2187.2 + (-2868) = -680.8 kcal
The correct answer was -340.4 kcal
Why do i have to divide by 2?QUOTE(befitozi @ Jul 19 2009, 04:30 PM) Shouldn't you be looking for energy of O=O bond instead of O-O? ya la... O2 is double bond, nt single bond... This post has been edited by vivienne85: Jul 19 2009, 07:07 PM |
|
|
|
|
|
befitozi
|
 Jul 19 2009, 07:15 PM Jul 19 2009, 07:15 PM
|

|
Pretty sure that you need to consider O=O though i maybe wrong because i'm pretty rusty with this.
Else, check the question, are they asking for Standard Enthalpy change of combustion or just enthalphy change of the reaction, if they are asking for standard enthalpy, means your jsut 1 step behind. Because standard enthalpy is defined as change per 1 mol of ethane. So just divide the energy change with the amount of mols of ethane which is 2.
EDIT: done some checking. the value you gave for O-O is infact the value for O=O. I missed that earlier because i did not realise that its in kcal. Therefore, just use the definition of standard enthalpy change.
This post has been edited by befitozi: Jul 19 2009, 07:48 PM
|
|
|
|
|
|
TSCKC_1
|
 Jul 19 2009, 08:59 PM Jul 19 2009, 08:59 PM
|

|
Here is the question 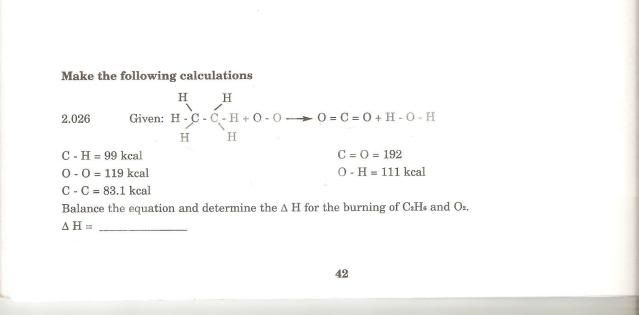 QUOTE Pretty sure that you need to consider O=O though i maybe wrong because i'm pretty rusty with this.
Else, check the question, are they asking for Standard Enthalpy change of combustion or just enthalphy change of the reaction, if they are asking for standard enthalpy, means your jsut 1 step behind. Because standard enthalpy is defined as change per 1 mol of ethane. So just divide the energy change with the amount of mols of ethane which is 2. I have to divide by 2 because the reaction requires 2 moles of C2H6 (ethane) right? how bout the 7 moles of Oxygen? does that affect the enthalpy in any way? This post has been edited by CKC_1: Jul 19 2009, 09:04 PM |
|
|
|
|
|
befitozi
|
 Jul 19 2009, 09:17 PM Jul 19 2009, 09:17 PM
|

|
QUOTE(CKC_1 @ Jul 19 2009, 08:59 PM) Here is the question  I have to divide by 2 because the reaction requires 2 moles of C2H6 (ethane) right? how bout the 7 moles of Oxygen? does that affect the enthalpy in any way? Look at it as the burning of 2mols of ethane, not a requirement of reaction. But the questions wants 1mol of ethane(imo the question i pretty vague, not specifying certain critical details), so you should balance the equation for 1mol of ethane. C2H6 + 3.5O2 --> 2CO2 + 3H2O you'll see that the now it will yield the -340kcal. |
|
|
|
|
|
TSCKC_1
|
 Jul 19 2009, 09:27 PM Jul 19 2009, 09:27 PM
|

|
okay got it now , thank you so much!
|
|
|
|
|
|
Cheesenium
|
 Jul 19 2009, 09:43 PM Jul 19 2009, 09:43 PM
|

|
This thread should be in Education Essentials.
It's related to TS's homework.
|
|
|
|
|
|
lin00b
|
 Jul 20 2009, 03:16 PM Jul 20 2009, 03:16 PM
|

|
but education essential says no homework help.
i'm beginning to feel the same should apply for science lab
|
|
|
|
|
|
befitozi
|
 Jul 20 2009, 05:34 PM Jul 20 2009, 05:34 PM
|

|
Well, TS's did not ask us to do his homework. His doubts and questions were very specific. Surely this kind of questions should be encouraged
|
|
|
|
|
|
Cheesenium
|
 Jul 20 2009, 09:21 PM Jul 20 2009, 09:21 PM
|

|
QUOTE(befitozi @ Jul 20 2009, 05:34 PM) Well, TS's did not ask us to do his homework. His doubts and questions were very specific. Surely this kind of questions should be encouraged TS didnt ask us to do his homework,but he was asking about specific part of it. I dont see whats wrong with posting it in EE. |
|
|
|
|


 Jul 19 2009, 12:49 AM, updated 17y ago
Jul 19 2009, 12:49 AM, updated 17y ago
 Quote
Quote

 0.2383sec
0.2383sec
 0.68
0.68
 5 queries
5 queries
 GZIP Disabled
GZIP Disabled