Outline ·
[ Standard ] ·
Linear+
Chemistry A few questions, need help please
|
befitozi
|
 Jul 19 2009, 04:30 PM Jul 19 2009, 04:30 PM
|

|
QUOTE(CKC_1 @ Jul 19 2009, 12:49 AM) Another question : I needed to do this calculation : I needed to balance the equation first (which i did already) and calculate ΔH 2(C2H6) + 7(O2) ---> 4(CO2) + 6(H2O) <<already balanced C-H = 99kcal
O-O = 119kcal
C-C = 83.1kcal
C=O = 192kcal
O-H = 111kcal
ΔH = ??
I did the calculation this way
Products = C-H 2( 99*6) + C-C 2(83.1) + O-O 7(119) = 2187.2 kcal
Reactants = O=C 4(192*2) + H-O 6(111*2) = -2868
2187.2 + (-2868) = -680.8 kcal
The correct answer was -340.4 kcal
Why do i have to divide by 2?Shouldn't you be looking for energy of O=O bond instead of O-O? |
|
|
|
|
|
befitozi
|
 Jul 19 2009, 07:15 PM Jul 19 2009, 07:15 PM
|

|
Pretty sure that you need to consider O=O though i maybe wrong because i'm pretty rusty with this.
Else, check the question, are they asking for Standard Enthalpy change of combustion or just enthalphy change of the reaction, if they are asking for standard enthalpy, means your jsut 1 step behind. Because standard enthalpy is defined as change per 1 mol of ethane. So just divide the energy change with the amount of mols of ethane which is 2.
EDIT: done some checking. the value you gave for O-O is infact the value for O=O. I missed that earlier because i did not realise that its in kcal. Therefore, just use the definition of standard enthalpy change.
This post has been edited by befitozi: Jul 19 2009, 07:48 PM
|
|
|
|
|
|
befitozi
|
 Jul 19 2009, 09:17 PM Jul 19 2009, 09:17 PM
|

|
QUOTE(CKC_1 @ Jul 19 2009, 08:59 PM) Here is the question 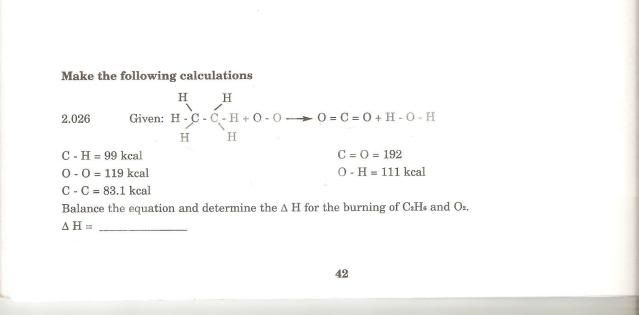 I have to divide by 2 because the reaction requires 2 moles of C2H6 (ethane) right? how bout the 7 moles of Oxygen? does that affect the enthalpy in any way? Look at it as the burning of 2mols of ethane, not a requirement of reaction. But the questions wants 1mol of ethane(imo the question i pretty vague, not specifying certain critical details), so you should balance the equation for 1mol of ethane. C2H6 + 3.5O2 --> 2CO2 + 3H2O you'll see that the now it will yield the -340kcal. |
|
|
|
|
|
befitozi
|
 Jul 20 2009, 05:34 PM Jul 20 2009, 05:34 PM
|

|
Well, TS's did not ask us to do his homework. His doubts and questions were very specific. Surely this kind of questions should be encouraged
|
|
|
|
|


 Jul 19 2009, 04:30 PM
Jul 19 2009, 04:30 PM

 Quote
Quote
 0.0164sec
0.0164sec
 0.58
0.58
 6 queries
6 queries
 GZIP Disabled
GZIP Disabled