I have a few questions in my chemistry book that I do not understand enough to move on to the next section . Can someone help me?
Reactions rates are affected by concentration ,collision geometry , and the presence of a catalyst . Which of the following statement is false concerning these factors.
a Increasing the concentration of reacting particles increases the chance for collision
b Optimum collision geometry lowers the activation energy barrier
c A reaction occurs each time particles of the reactants collide.
d The slowest reaction incolved in a reaction mechanism determines the rate of overall reaction
the correct answer is c ,but why? I did alittle research online and found this statement "A chemical reaction occurs when substances (the reactants) collide with enough energy to rearrange to form different compounds (the products)."
isnt d correct" why then is c false?
Can someone also explain B and D?
Another question :
I needed to do this calculation :
I needed to balance the equation first (which i did already) and calculate ΔH
2(C2H6) + 7(O2) ---> 4(CO2) + 6(H2O) <<already balanced
C-H = 99kcal
O-O = 119kcal
C-C = 83.1kcal
C=O = 192kcal
O-H = 111kcal
ΔH = ??
I did the calculation this way
Products = C-H 2( 99*6) + C-C 2(83.1) + O-O 7(119) = 2187.2 kcal
Reactants = O=C 4(192*2) + H-O 6(111*2) = -2868
2187.2 + (-2868) = -680.8 kcal
The correct answer was -340.4 kcal
Why do i have to divide by 2?
Chemistry A few questions, need help please


 Jul 19 2009, 12:49 AM, updated 17y ago
Jul 19 2009, 12:49 AM, updated 17y ago
 Quote
Quote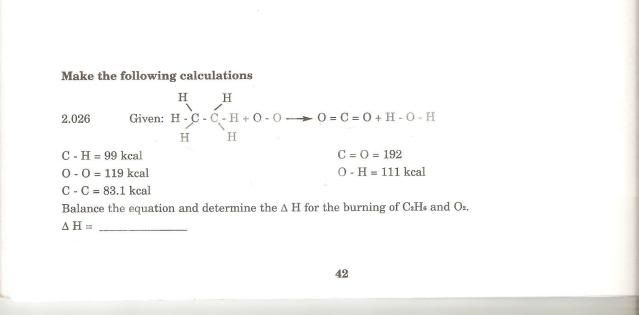
 0.0155sec
0.0155sec
 0.51
0.51
 6 queries
6 queries
 GZIP Disabled
GZIP Disabled